Topics to be discussed (As per H.S final year Chem. Syllabus 2022-23)
- Definition of solid .
- Amorphous and crystalline solids
- Classification of solids based on the nature of particles and binding forces: Ionic solids, molecular solids, covalent and metallic solids
- Unit cells in 2D and 3D lattice.
- Types of unit cells on the basis of number and location of particles.
- Calculation of no. of atoms per unit cell.
- Relationship between edge length of unit cell and radius of particles
- Packing in solids, CCP and HCP
- Packing fraction & its calculation for each type of unit cell.
- Voids and their types,
- Nearest neighbour distance and co-ordination number.
- Calculation of density of unit cell.
- Defects in solids (only point defects)
Definition of solid: A solid is defined as that form of physical state, which possesses rigidity and hence possesses definite shape and definite volume.
Based on the arrangement of constituent particles, solid substances are broadly classified into two main categories: a) Amorphous solid and b) Crystalline solids.
Amorphous Solid: A solid is said to be amorphous if there is no regular geometric arrangement of constituent particles. For example rubber, plastic, polymers of high molecular mass etc. exist in this form.
Crystalline solid: A solid is said to be crystalline if its constituent particles are arranged in a definite geometric pattern in three dimensional space. For example, all solid elements of metal and non-metals and their compounds exist in this form.
Some important properties of solids are mentioned below:
Isotropy : In the case of amorphous solids, properties like electrical conductivity, refractive index, thermal expansion etc are identical in all directions. This property is called Isotropy and the solid substance which possesses this property is called Isotropic solid.
Anisotropy: In the case of crystalline solids, properties like electrical conductivity, refractive index, thermal expansion etc have different values in different directions. This property is called Anisotropy and the solid which possesses this property is called Anisotropic solid.
Distinguish between crystalline and amorphous solids :
- In a Crystalline solid particles are arranged in a definite geometric pattern. On the other hand, in an amorphous solid particles are not arranged in a definite geometric pattern.
- Crystalline solids are anisotropic in nature. On the other hand, amorphous solids are isotropic in nature.
- Crystalline solids have a definite heat of fusion. On the other hand amorphous solids do not have definite heat of fusion
- Crystalline solids have very sharp melting points. On the other hand amorphous solids melt over a range of temperatures.
- Crystalline solids undergo a clean cut. On the other hand, amorphous solids undergo irregular cuts.
- Crystalline solids are true solids, On the other hand amorphous solids are considered as pseudo solids or super-cooled liquids.
Classification of crystalline solids: On the basis of the nature of particles and binding forces, crystalline solids are further classified into four categories:
- Ionic solids.
- Molecular solids.
- Covalent or network solids.
- Metallic solids.
Ionic Solids
Nature of Constituent particles: The constituent particles of ionic solids are ions i.e cations and anions.
Nature of binding force: The oppositely charged ions of an ionic solid are held together by a strong electrostatic force of attraction
Main characteristics :
- They have very high melting points and boiling points.
- Explanation: Due to the strong electrostatic force of attraction between the oppositely charged ions, more heat is required to overcome the inter–ionic force of attraction.
- They are conductors of electricity in a fused state, but they are insulators in solid state.
- Explanation: In a fused state they undergo dissociation to form free ions. Due to the presence of these free ions they conduct electricity. But in the solid state, they don’t undergo dissociation to give free ions. Due to the absence of free ions they can’t conduct electricity.
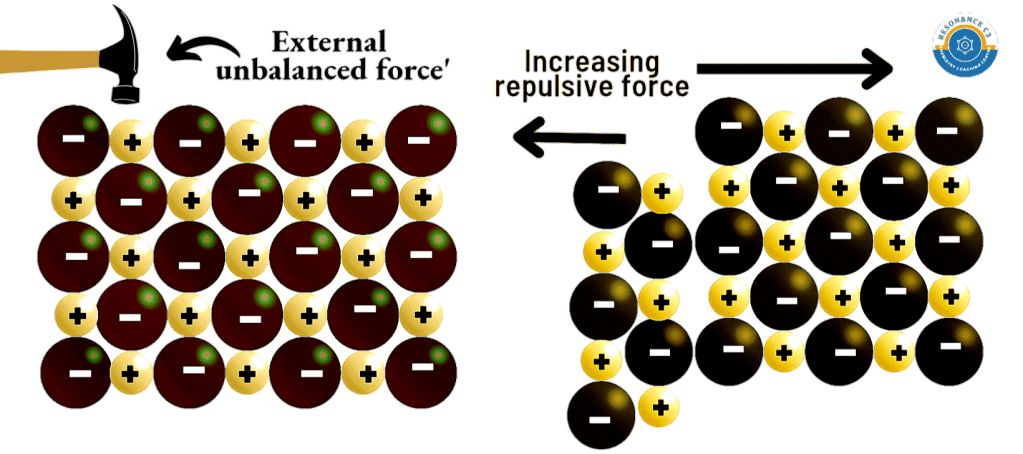
- Ionic solids are hard and brittle.
- Explanation: Hardness is because of the strong electrostatic force of attraction, which holds the ions together firmly. But when an external force is applied on the crystal, the ions slide a bit and in doing so the ions of similar charges come closer and begin to repel one another. This tendency breaks the crystal easily.
- Ionic solids are water soluble but they are insoluble in organic solvents like benzene, carbon tetrachloride etc.
- Explanation: Since polar solutes are soluble in polar solvents and insoluble in non-polar solvents , hence being polar in nature Ionic solids get dissolved in water which is a polar solvent. But organic solvents like benzene, carbon tetrachloride etc are being non-polar and can’t dissolve polar solutes like ionic solids.
Molecular Solids
Molecular solids contain molecules as their constituent particles. The binding forces between the molecules are London dispersion forces, Dipole-dipole interaction and Hydrogen bonding.
Depending upon the nature of binding forces molecular solids are further classified into three sub- categories: Non-polar Molecular Solids
- Non-polar Molecular Solids
- Polar Molecular Solids
- Hydrogen Bonded Molecular Solids
a) Non-polar Molecular Solids:
Nature of constituent particles: The constituent particles of these types of solids are non-polar molecules like, diatomic molecules of hydrogen (H2), chlorine (Cl2), Iodine (I2) etc.
Nature of Binding force: The binding force which holds the non-polar constituent particles is London dispersion force.
Main Characteristics:
- These solids are soft in nature because of the weak London forces of attraction.
- Due to the soft nature, they possess low melting and boiling points.
- They are non-conductors of electricity due to the absence of free ions.
- Due to weak forces of attraction they exist as gases at ordinary temperatures.
b) Polar Molecular Solids
Nature of Constituent particles: Constituent particles of these types of solids are the polar molecules like hydrogen chloride, sulphur dioxide etc.
Nature of binding force: The constituent particles of these types of solids are held together by Dipole-dipole force of attraction, which is stronger than London dispersion forces.
Main Characteristics
- They are soft in nature
- They are non-conductors of electricity due to the absence of free ions.
- Their melting points and boiling points are higher than that of non-polar solids.
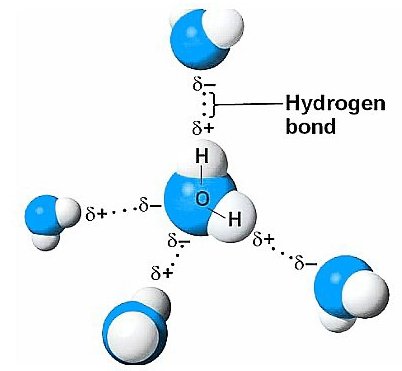
c) H – Bonded Molecular Solids
Nature of particles: The constituent particles of these types of solids are those molecules which can form Hydrogen bonding between them. For example: Water, ammonia etc.
Nature of binding force: The molecules of these solids are held together by intermolecular H-Bonding .
Main Characteristics:
- They are volatile liquids or soft solids at normal temperature.
- They are non-conductors of electricity. Reason: Due to the absence of free ions they can’t conduct electricity
- Their melting points are comparatively higher than that of polar molecular solids and Non-polar molecular solids, Reason: Since H-Bonding is stronger than dipole-dipole interactions and London dispersion forces.
Covalent Solids
Nature of Constituent Particles: The constituent particles of covalent solids are ‘atoms.’
Nature of binding forces: The constituent particles of these types of solids are held together by strong ‘covalent bond force’ .
Example of covalent solids are Diamond, Graphite, Silicon Carbide, Quartz , Boron nitride etc.
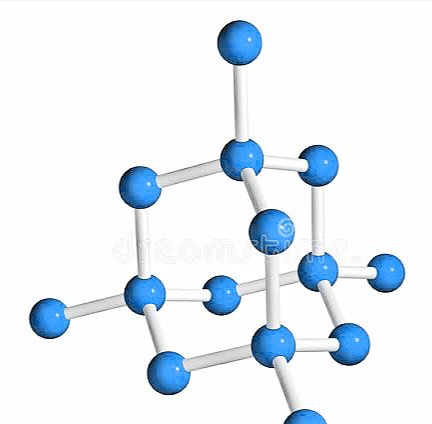
Structure of Diamond:
Each carbon atom of diamond is sp3 hybridized. In the crystal lattice of diamond each carbon atom forms four covalent bonds with other four carbon atoms to form a tetrahedral structure. Since all valence electrons are involved in bond formation, so there is no any free electrons in the crystal lattice of diamond. Due to the absence of free electrons diamond can’t conduct electricity.
Structure of Graphite:
Each carbon atom present in the crystal lattice of Graphite is sp2 hybridized. So each carbon atom forms three covalent bonds with the other three carbon atoms to form hexagonal layered structure. Each carbon contains one free electron in the pure p-orbital. Due to the presence of free electrons it becomes good conductor of electricity.
Two hexagonal layer of sp2 carbon atoms are separated by Vander Waal’s distance. The force of attraction that holds the layers together is very weak in nature. As a result of weak force each layer can slide over another layer. This property makes the crystal slippery in nature. Due to this slippery nature graphite is used as a lubricant in machinery parts.
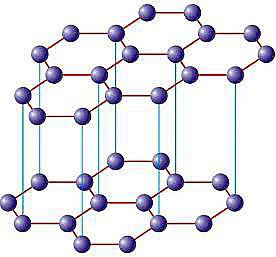
Main Characteristics
- They are generally hard due the presence of strong covalent bond force
- They have high heat of fusion
- Due to strong covalent forces covalent solids have very high melting points and boiling points.
- Covalent solids are bad conductors of electricity due to the absence of free electrons in their crystal lattices. (Exception ——> Graphite)
Metallic solids
Nature of particles: Positively charged charged cations and free electrons of the metal.
Nature of binding force: The constituent particles of a metallic solid are held together by strong metallic bonds.
Origin of metallic bonds: In the crystal of a metallic solid, metal atoms release their valence electrons which are loosely held by their respective nuclei, due to their low ionisation enthalpy energy. The free electrons of the metal can move freely in throughout the crystal all directions, like water in the sea. Hence it is also called Sea of free electrons.
The mobile electrons are simultaneously attracted by posotive charged cations, and hence holds positively ions together. The force that holds the metal ions together in the crystal is called metallic bond,
Main Characteristics
- Metallic solids are very good conductors of electricity and heat. (Thermal conductors) . Reson for electrical conductivity: Since the crystal of a metal contains free electrons. When electric field is applied across the metallic solid, each electron starts moving from lower potential region to higher potential region. Thus they conduct electricity. Reason for thermal conductivity: When heat is supplied to one end of the metallic solid, the free electrons absorbs the heat energy to all parts of the metallic solid.
- They possess lustre and colour in cases. This property can also be explained on the basis of the ‘Sea Theory’ of metallic solid.
- They are malleable and ductile:। Reason: In metallic solids, the positions of cations can be altered without destroying the crystal because of uniform charge distributions provided by freely moving electrons. For this reason metallic solids can be deformed.
Space lattice or crystal lattice
Space lattice is a regular repeating arrangement of points . If we consider a small part of the lattice which when repeated in different directions produces the complete space lattice.
Two dimensinoal lattices: A two dimensinoal lattice is a regular arrangment of points in the plane of the paper. We suitably choose four ponts. and connect them to obtain the unit cell.
Types of two dimensinoal lattices
- Squre lattice
- Rectangular lattice
- Parallelogram lattice
- Rhommbic
- Hexagonal lattice.
Three dimensional crystal lattice
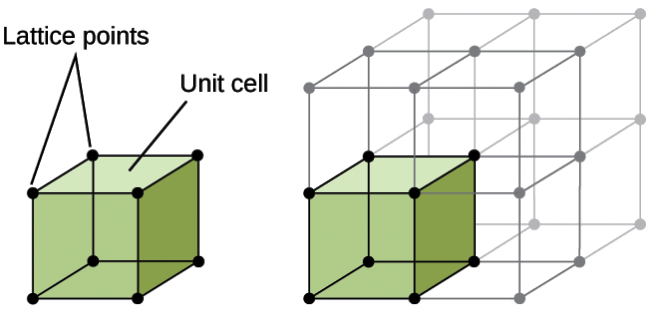
Crystal lattice: The constituent particles of a crystalline solid are arranged in definite geometric pattern in the three dimensional space. Such a regular arrangment of particles is called Crystal lattice.
Some characteristics of a crystal lattice:
- Each point in the crystal lattice represents a constituent particle which may be an atom , a molecule or an ion.
- Each point in the lattice is called lattice point or lattice site.
- The points are joined by lines just to represent the geometry of the lattice.
Unit Cell: The three dimensional portion of a complete space lattice which when repeated over and again in different directions produces the complete space lattice is called the unit cell.
Types of unit cells: Based on the positions of particles present, unit cells are broadly classified into two main categories: a) Primitive unit cells or simple unit cells b) Non – primitive or centred unit cells.
Primitive unit cells: The unit cells in which the constituent particles are present only at the corners are called primitive unit cells.
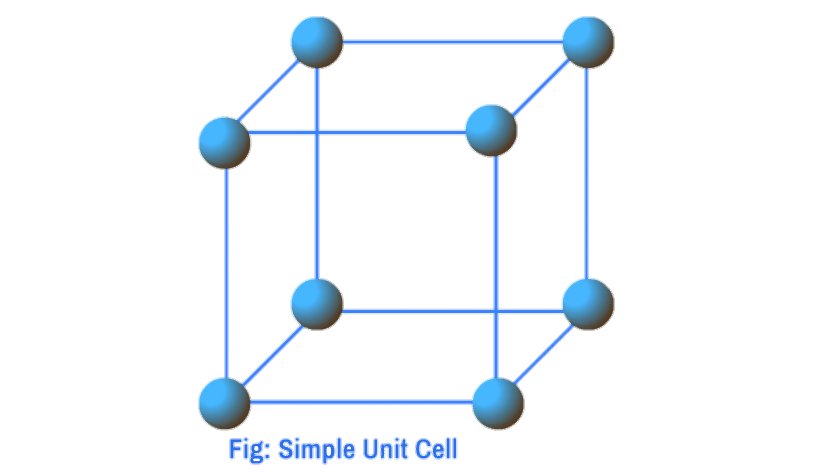
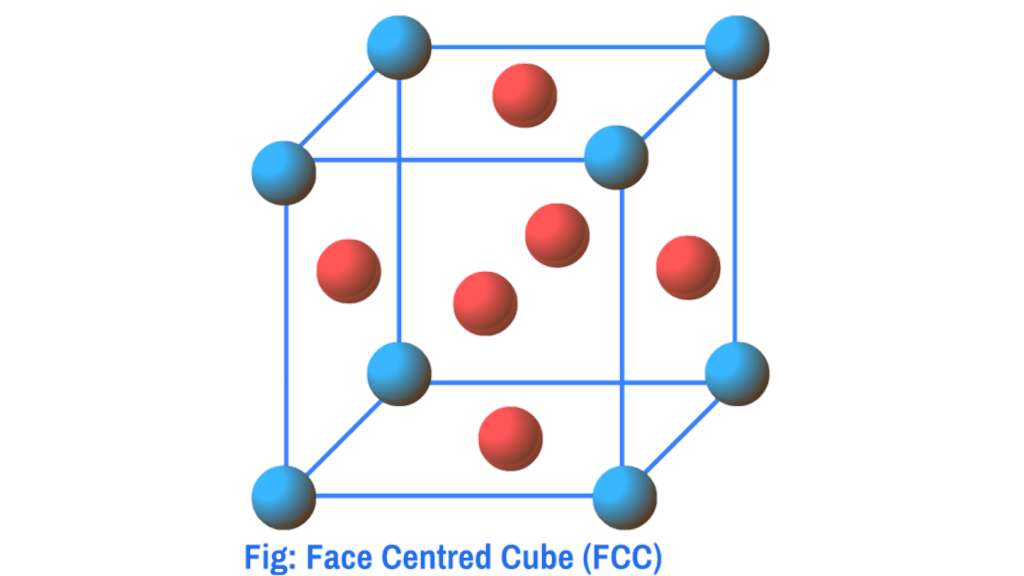
Non – primitive unit cells: The unit cells in which constituent particles are present not only at the corners of the unit cells but also at some other positions are called Non – primitive unit cells . Non – primitive unit cells are further classified into three categories:
- Face Centred Cube (FCC)
- Body Centred Cube (BCC)
- End Face Centred Cube (EFCC)
Face Centred Cube (FCC) : When in a unit cell constituent particles are present not only at the corners but also at the centre of each face the unit cell is called face-centred cube. (fcc)
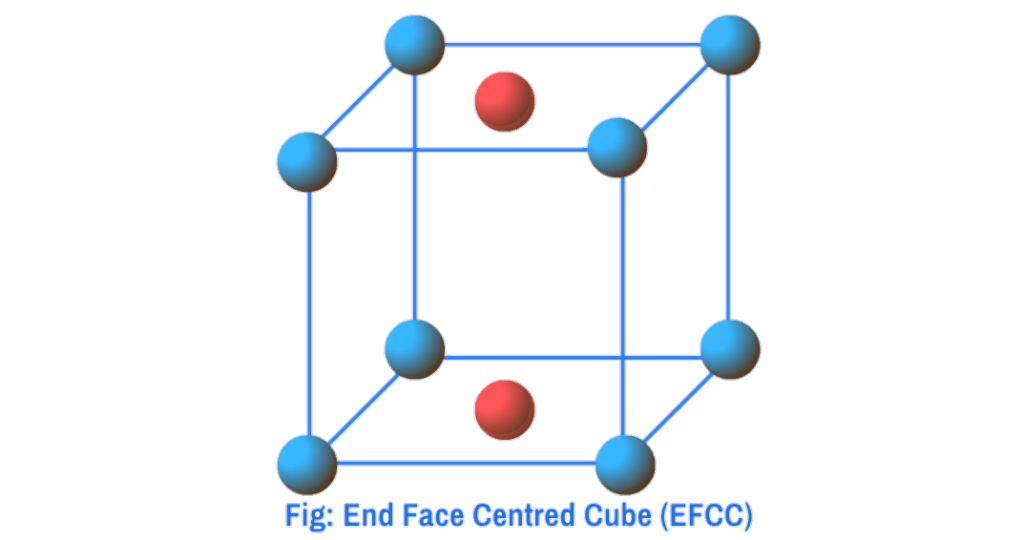
Body Centred Cube (BCC) : When in addition to the particles at the corners, there is one particle present at the centre within the body of the unit cell, it is called body-centred cube (bcc)
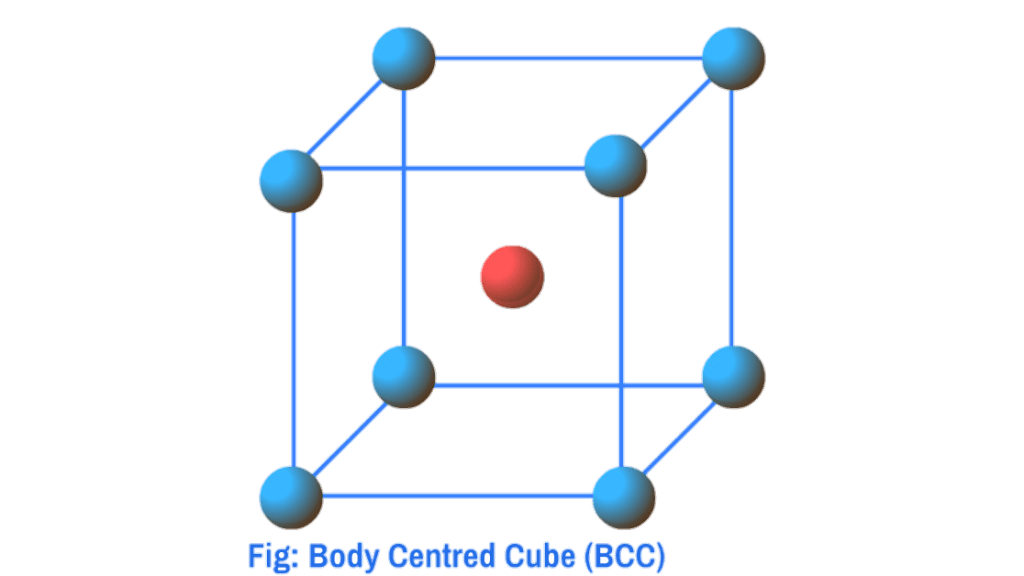
End-face Centred Cube (EFCC) : When in addition to the particles at the corners, there are particles at the centres of any two opposite faces, it is called End-face centred cube (efcc)
Calculation of number of particles present per unit cell:
a) For Simple Cubic Cells:

In the case of a simple cubic cell , particles are present at the corners only. Each corner particle is equally shared by eight unit cells. As a result contribution of one corner particle is 1/8, and the total contribution of eght corners paticles is 8×1/8 = 1. Thus a simple cubic cell contains 1 particle only.
b) Face Centred Cube (FCC)
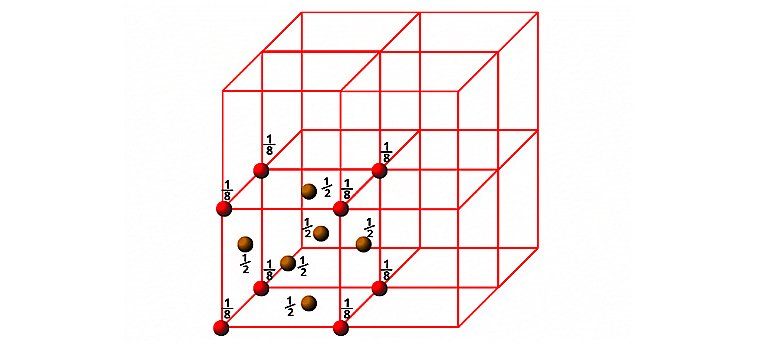
In the case of fcc , particles are present at the corners as well as face centres. Since each corner particle is equally shared by eight unit cells, so contributions from eight corners is equal to 8× 1/8=1. Similarly, since each face centred particle is equally shared by two unit cells, so contributions from six faces = 6×1/2= 3. Therefore, total number of particles present in the fcc unit cell= 1+3=4.
c) Body Centred Cube (BCC)
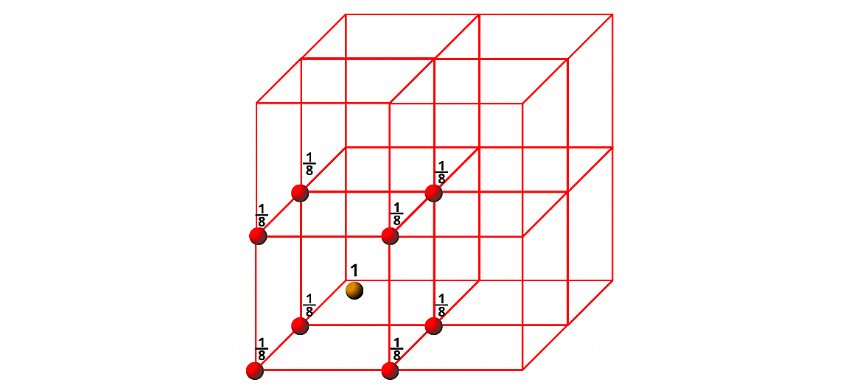
In the case of bcc, particles are present at the corners and one particle is present at the body centre of the unit cell. Since each corner particle is equally shared by eight unit cells so contributions from eight corners = 8×1/8 = 1, and since the body centred particle is not shared by any other unit cells so contribution of body centre = 1. Thus total number of particles present per bcc unit cell is 1+1=2.
d) End Face Centred Cube (EFCC)
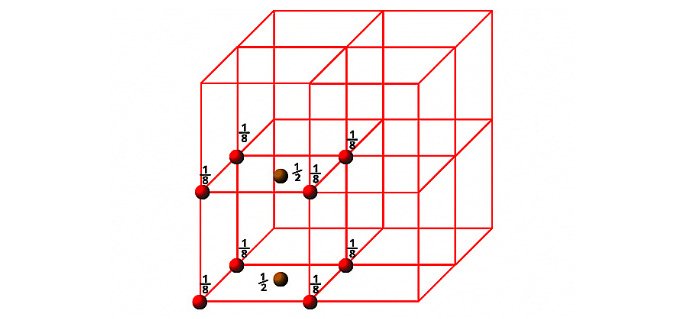
In the case of End-face Centred Cube, in addition to the corner particles there is one particle at the centre of any two opposite faces of the unit cell. Since each corner particle is equally shared by eight unit cells, so contributions from eight corners = 8×1/8 = 1. Similarly, each face centred particle is equally shared by two unit cells, so contributions from two opposite faces is equal to 2×1/2= 1 . Therefore, total number of particles present in an EFCC = 1+1=2.
Relationship between the edge length of a unit cell and radius of particle (Sphere):
For Simple Cubic Cell:
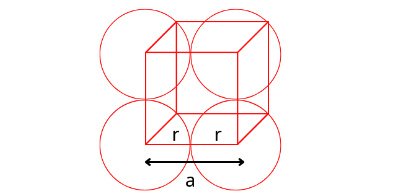
In the case of simple cubic cell, each corner particle is touched by two other corners paticles along the edge length. If each corner particle is represented by a sphere whose radius is ‘r’ and edge length of each edge is ‘a’ , then we can write a = r+r = 2r. or r = a/2

For Face Centred Cube (FCC):

In the case of fcc, the face centred particle touches the corners particles of the same face along the direction of the face diagonal. In the above diagram the face diagonal AB = r + r + r + r = 4r ………….(1) Again from the right angled triangle ABC, the value of diagonal AC can be calculated by Pythagoras theorem, AB² = AC² + CB² therefore, AB² = a²+a² = 2a² => AB = √2a.…………(2)
Now from equations 1 & 2 we get , 4r = √2a, so , r = √2a/4= a/2√2

For Body Centred Cube (BCC):
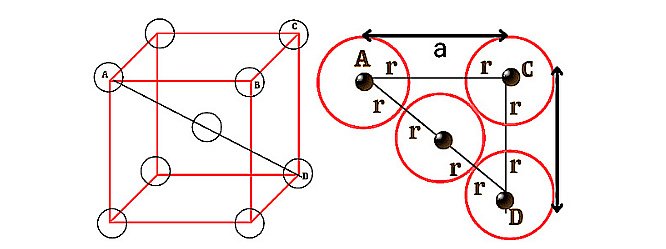
In the case of bcc, the body centred particle touches all the corner particles along the direction of body diagonal. In the above diagram, the value of the body diagonal AD = r+r+r+r = 4r………..(1). If we consider a right angled triangle ABC in the above unit cell, then the face diagonal of the triangle AC²=AB² + BC² => a² + a² = 2a² therefore AC = √2a. Again if we consider the right angled triangle ACD, then the value of its body diagonal AD will be AD² = AC² + CD² => AD² = (√2a)² + a² => AD²= 2a² + a² => AD = √3a ………………(2)
From equations 1 & 2 we get, 4r = √3a, therefore, r = √3a/4.
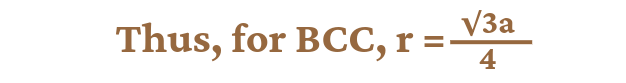
Packing fraction or Packing Efficiency
Packing fraction: The percentage of the total space filled by the particles is called Packing Fraction or Packing Efficiency . Mathematically, it is the ratio of volume of particles to the total volume of the unit cell. i.e packing fraction= volume of particles ÷ volume of the unit cell.
Calculation of packing fraction:
Simple Cubic Cell: In the case of a simple cubic cell , suppose the edge length of the unit cell is ‘a’ and ‘r’ is the radius of the particle, then a = 2r. The volume of a spherical particle v′ = 4/3πr³ => v‘ = 4/3 π (a/2)³ => v‘ = a³π/6. Now volume of the unit cell V = a³
Now packing fraction= v‘/V => a³π/6/a³ = π/6 = 0.524 = 52%
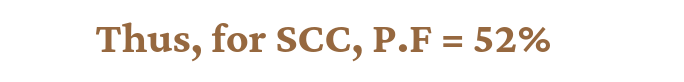
For Face Centred Cube (FCC): In the case of fcc, there are four particles present per unit cell. Since volume of one spherical particle= 4/3πr³ , so volumes of four spherical particles will be v‘ = 4× 4/3πr³ => 16π/3 (a/2√2)³ . (Since for fcc r= a/2√ 2) . Therefore v‘= 16π/a³/16√2. ……. (1)
Again volume of the unit cell. (V) = a³…… (2). Now dividing equation 1 by equation 2, we get , packing fraction= 16πa³/16√2a³ = 0.74 = 74%

Voids in solids
Voids are the empty spaces formed in the lattice structure of solid
Numerical Problems based on the number of particles present per unit cell:
Defects in Solids
Definition: Any departure from perfectly ordered arrangements of constituent particles in the crystal is called defect or imperfection in solid.
Cause of defects: Defect in solid may arise due to the heat absorption by the crystal or due to the presence of impurities present in the crystal.
Defects are broadly classified into three main categories: a) Point defect and b) Line Defect. But our syllabus contains only the point defect. So here we will study about the point defect only.
Point Defect
When the deviations or irregularities in the ideal arrangement of constituent particles around a point or atom in the crystal, it is called point defect. Point defects are further classified into three categories:
- Stoichiometric defect
- Non – Stoichiometric defect
- Impurity defect
Stoichiometric defects: When imperfections in the solid are such that the ratio between the number of cations and anions is same as represented by the molecular formula of the solid i.e the stoichiometry of the solid is not disturbed, are called stoichiometric defects.
These defects are also called intrinsic defects or thermodynamic defects. These are also two types: a) Vacancy defects b) Interstitial defects.
Vacancy defects: When in a crystalline solids some particles from the latice sites are missing, but the stoichiometric rato between the particles is not disturbed, it is called vacany defect.
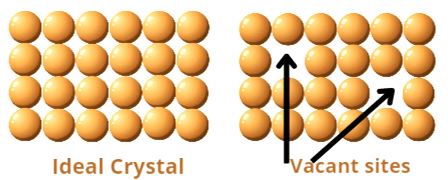
Consequences of vacancy defects : Due to vacancy defects mass of the crystal is decreased but volume of the unit cell remains unchanged. As a result of which, density of the unit cell decreases.
Non – Stoichiometric defects:
